
The faulty explanation is incorrect even if restricted to chlorine, bromine and iodine: Although the ease with which an atom attracts an electron matters, it is not as important as the hydration enthalpy of the negative ion formed. Down the group, the ions become less attractive to water molecules as they get larger. Using the figures from the previous table:īoth of these effects contribute, but the more important factor-the one that changes the most-is the change in the hydration enthalpy.
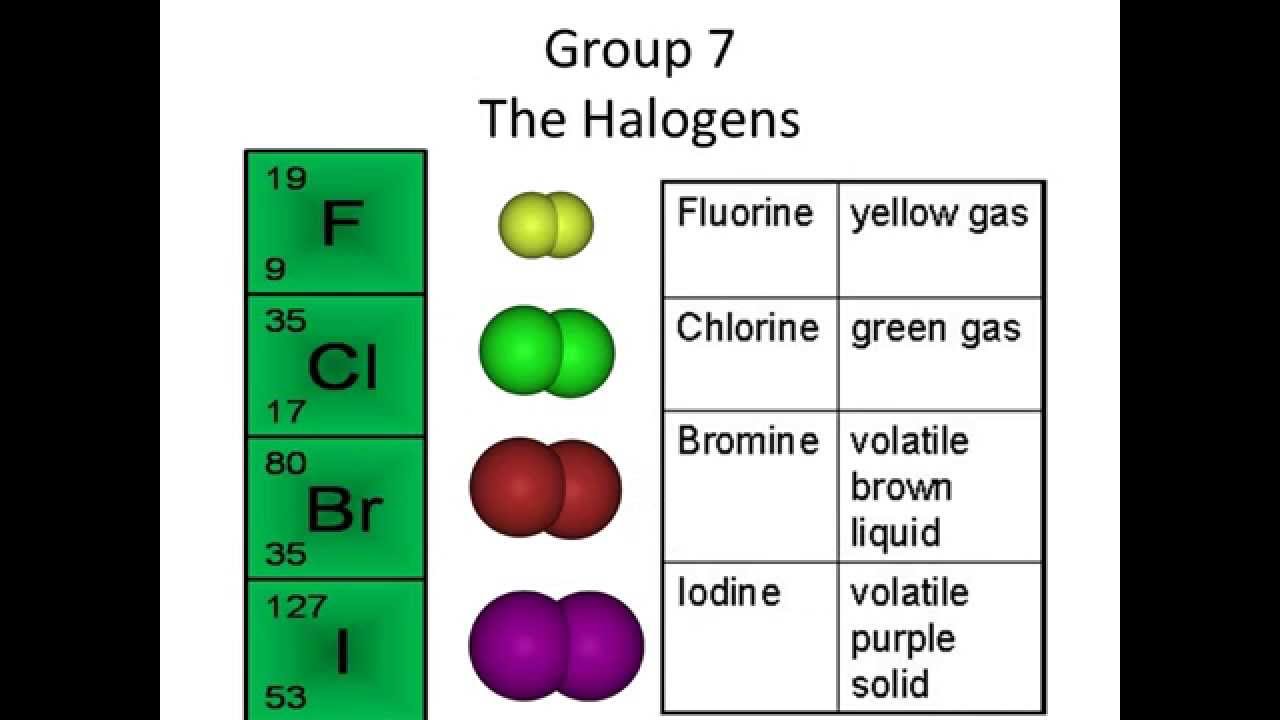
It is helpful to look at the changes in electron affinity and hydration enthalpy down the group. The decrease in atomization energy between these three elements is relatively small, and would tend to make the overall change more negative down the group. Why does oxidizing ability decrease from chlorine to bromine to iodine? The fifth column measures the energy released when 1 mole of gaseous ions dissolves in water to produce hydrated ions, as in the following equation, which is not equivalent to that above: The first electron affinity is defined as the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions, as in the following equation: In symbol terms: Fluorine generates a large amount of heat when it forms its hydrated ion, chlorine a lesser amount, and so on down the group. The amount of heat evolved decreases quite dramatically from the top to the bottom of the group, with the biggest decrease between fluorine and chlorine. The table below shows the energy involved in each of these changes for atomization energy, electron affinity, and hydration enthalpy (hydration energy):Ĭonsider first the fifth column, which shows the overall heat evolved, the sum of the energies in the previous three columns. The isolated ions are surrounded by water molecules hydrated ions are formed (hydration).Each atom gains an electron (electron affinity this is the element of the process of interest in the faulty explanation.).The diatomic molecule must split into individual atoms (atomization).This may be a gas, liquid or solid at room temperature, depending on the halogen. The halogen starts as a diatomic molecule, X 2.The argument about atoms accepting electrons applies only to isolated atoms in the gas state picking up electrons to form isolated ions, also in the gas state. This problem stems from examining a single part of a very complicated process. However, fluorine's electron affinity is less than that of chlorine. Fluorine's tendency to form a hydrated ion is much higher than that of chlorine. The problem with this argument is that it does not include fluorine.

Electron affinity is described in detail on another page. This is equivalent to saying electron affinity decreases down the group. The larger atoms are therefore less effective at attracting new electrons and forming ions. As the atoms get larger, the new electrons are further from the nucleus and increasingly shielded by the inner electrons (offsetting the effect of the greater nuclear charge). The ease of ionization depends on how strongly the new electrons are attracted. The following explanation is normally given for the trend in oxidizing ability of chlorine, bromine and iodine. Electron Shielding increases and outweighs the nuclear attraction.\) Nuclear charge increases, so the nuclear attraction on the outer electrons are stronger. Volatility decreases down the group as the boiling points increase.Īs you go down group 7, the halogens become less reactive. This trend is highlighted by the fact that the physical state of the halogens changes from gaseous (fluorine) to solid (iodine) down the group. This is because the strength of the Van Der Waals forces (or induced dipole-dipole interactions) increases since the atoms have more electrons as you descend the group. The boiling and melting points increase as you go down the group. Some main properties of the first 4 elements in group 7 are listed below.ġs 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 5
Group 7 consists of highly reactive non-metals called halogens.


 0 kommentar(er)
0 kommentar(er)
